Rows: 1,314
Columns: 13
$ seqn <dbl> 83734, 83750, 83754, 83757, 83789, 83790, 83799, 83809, 83813…
$ riagendr <fct> Male, Male, Female, Female, Male, Male, Female, Female, Male,…
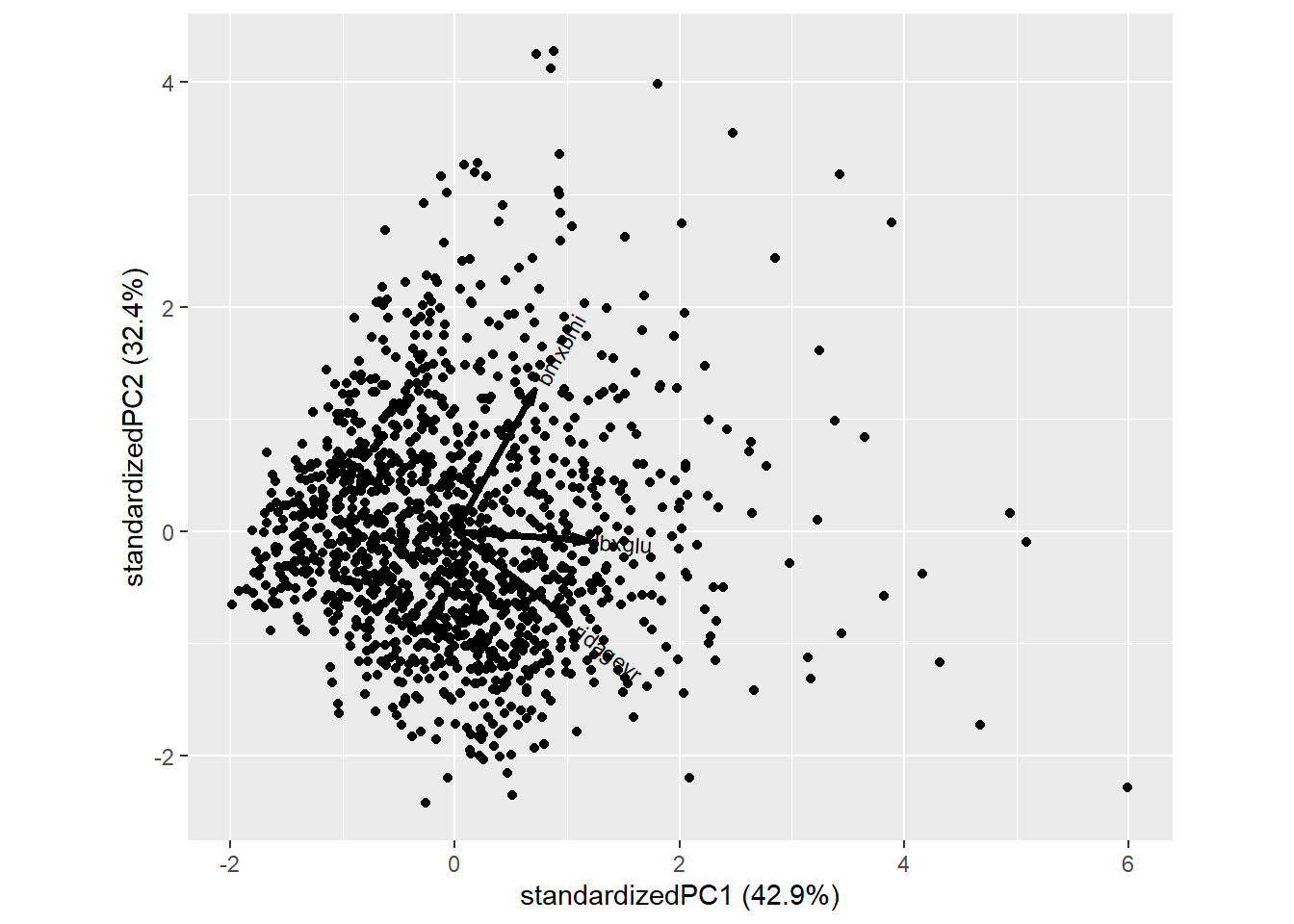
$ ridageyr <dbl> 78, 45, 67, 57, 66, 56, 37, 20, 24, 80, 29, 39, 35, 40, 54, 7…
$ ridreth1 <fct> Non-Hispanic White, Other, Other Hispanic, Other Hispanic, No…
$ dmdeduc2 <fct> High school graduate/GED, Grades 9-11th, College grad or abov…
$ dmdmartl <fct> Married, Never married, Married, Separated, Living with partn…
$ indhhin2 <fct> "$20,000-$24,999", "$65,000-$74,999", "$25,000-$34,999", "$20…
$ bmxbmi <dbl> 28.8, 24.1, 43.7, 35.4, 34.0, 24.4, 25.5, 26.2, 26.9, 28.5, 2…
$ diq010 <fct> Diabetes, No Diabetes, No Diabetes, Diabetes, No Diabetes, No…
$ lbxglu <dbl> 84, 84, 130, 398, 113, 397, 100, 94, 105, 119, 102, 101, 97, …
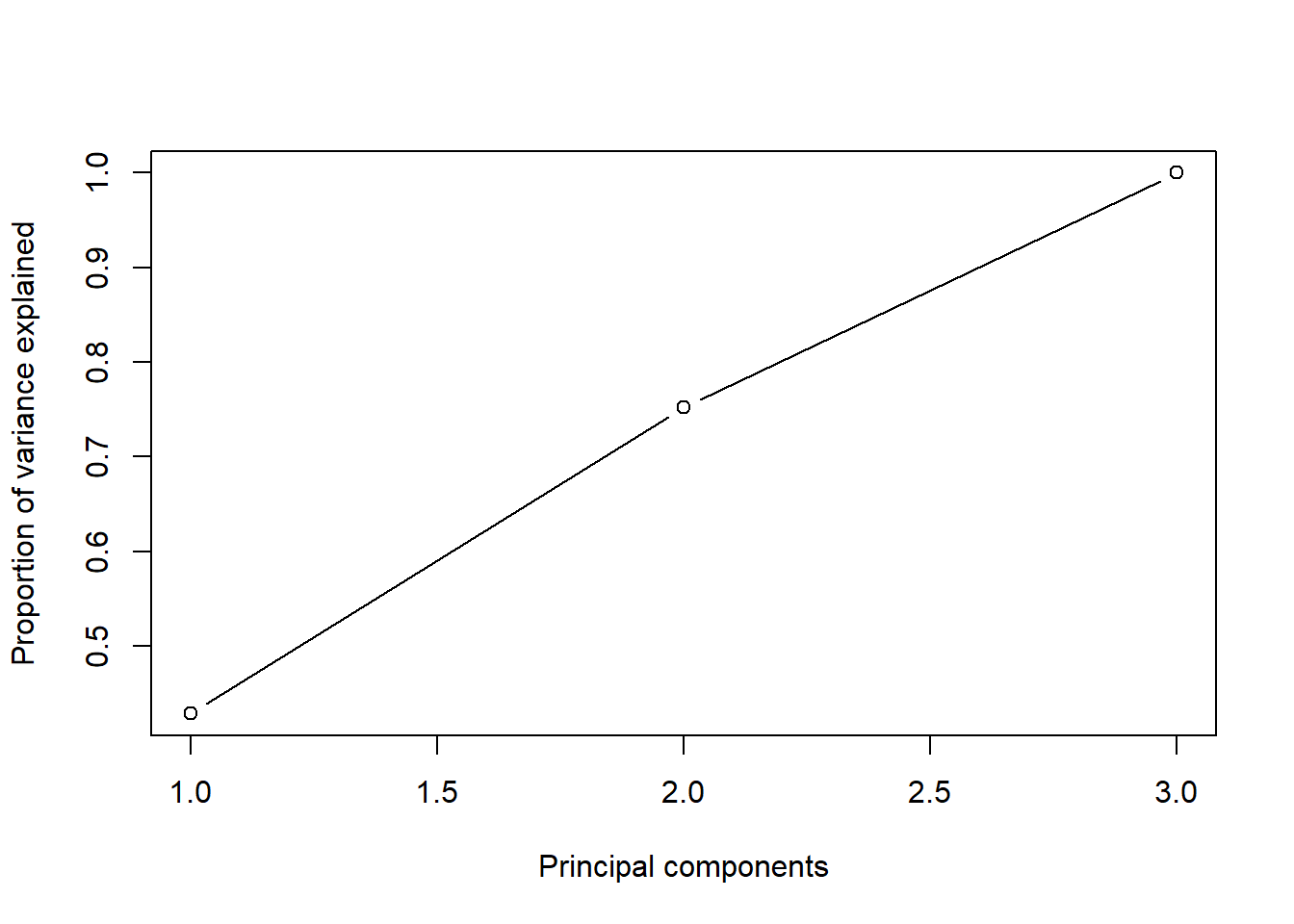
$ PC1 <dbl> 0.39364202, -1.01945675, 1.71575141, 5.60741419, 0.80332766, …
$ PC2 <dbl> -0.8830738811, -0.4475439639, 1.2070666916, 0.1554659373, 0.0…
$ PC3 <dbl> -1.465045765, -0.092975735, -0.945046444, 4.782660350, -0.759…